“As a biotech start-up company, we knew we had to lean heavily on BDD’s expertise and disciplined processes to navigate the complexities of MHRA regulatory submission, manufacture radiolabeled material, manage complexity of a combination product, and the execute a meaningful, 5-way crossover, PK/PD study. The seamless team dynamic and expertise at managing critical vendors, while communicating clearly and with precision throughout truly set them apart. After more than 35 years of running Phase 1 trials, I can confidently say this team ranks among the best in the Phase 1 space.” – David Skarinsky – SVP Clinical Development





















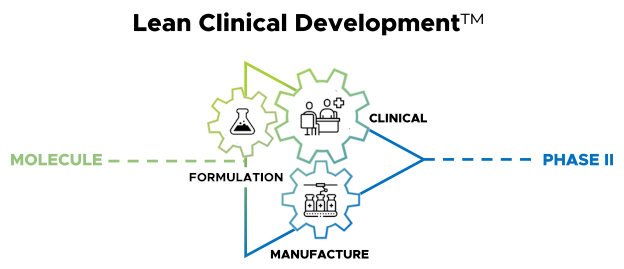









Positive Phase 1b Results Demonstrate Precise Timed Delivery for Contera Pharma’s CP-012 Using BDD’s OralogiK™ Technology