Rigorous Safety Measures: Implementing strict protocols to protect trial participants. Based on site within the grounds of a large teaching hospital with immediate access to 24-7 cover from the hospital medical emergency team and within 10 minutes of an intensive care unit.
Detailed Pharmacokinetic Analysis: Through BDD’s preferred partners we offer PK analysis showing how a drug is absorbed & metabolized by the body.
Informed FIH Trial Design: Using BDD expertise to help and support with clinical trial design to maximise study success.
Strategic Dose Selection: We have an expert and experienced FIH PI who leads our team with dose selection and we regularly update & review with data review meetings. Leveraging our expertise to increase the likelihood of success.
Data management: BDD can offer in house data management services, can work with a Sponsor preferred EDC or introduce clients to our partner agencies. Through our partner agencies data can be provided in CDISC format if required.
Statistics: BDD can provide FIH and phase 1 statistical packages through partner agencies
Special Population Trials:
- Healthy Volunteers and Patients: We conduct trials that span various populations, from healthy volunteers to specific patient groups such as those with respiratory conditions, diabetes, and gastrointenstinal diseases. This diversity in trial populations helps gather relevant data across different cohorts, providing a robust understanding of the investigational drug’s safety and efficacy.
Detailed Examples:
- Elderly and Special Conditions: We have managed trials involving post-menopausal females, patients over 60, and individuals with chronic conditions such as COPD and type I Diabetes. Our tailored approaches ensure that these trials meet the unique needs of each population, providing insights that are critical for developing safe and effective treatments.
Emphasising Safety and Quality:
Safety and quality are paramount in our FIH trials. We implement rigorous risk management strategies, including continuous risk-benefit analysis and clearly defined stopping rules for individual subjects, cohorts, and the entire study. Our protocols are designed to ensure participant safety at every step, from initial dosing to follow-up assessments.
Regulatory Compliance and Engagement:
We maintain proactive engagement with regulatory agencies such as the MHRA to ensure our trials comply with the highest standards. Our team’s extensive experience in navigating regulatory landscapes ensures smooth and compliant trial processes, minimizing delays and optimizing outcomes.
Technological and Methodological Innovations:
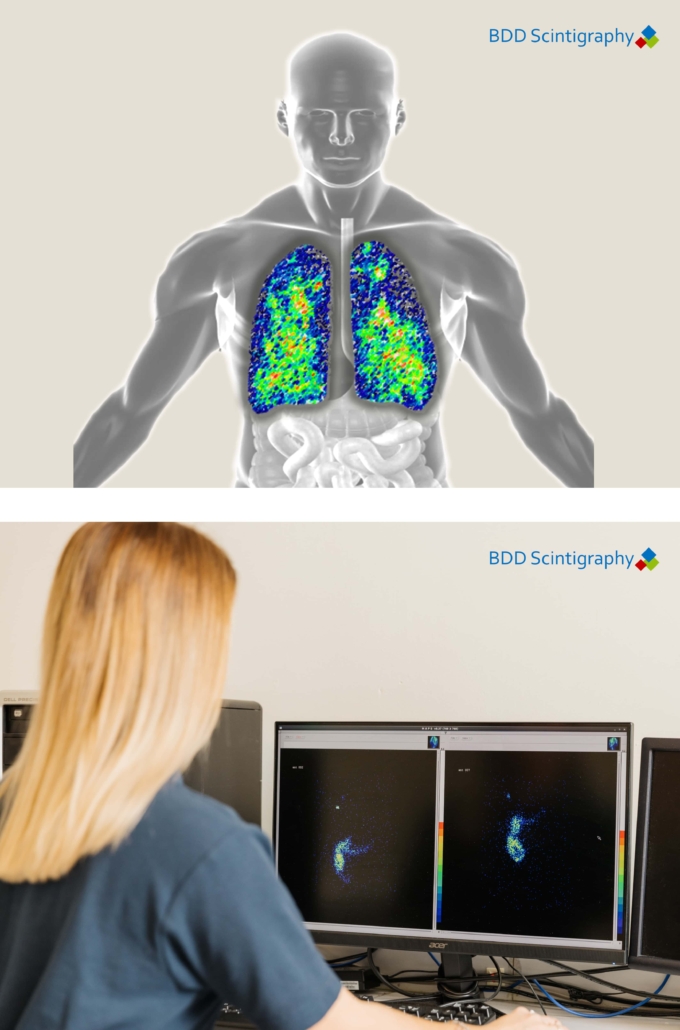
We leverage advanced technologies and innovative methodologies to enhance the quality and reliability of our trial data. This includes the use of cutting-edge imaging techniques, such as gamma scintigraphy, and on-site laboratory capabilities that provide rapid, high-quality data analysis.