Eliminating Unnecessary Steps with Lean Clinical Development™
Eliminating Unnecessary Steps with Lean Clinical Development™
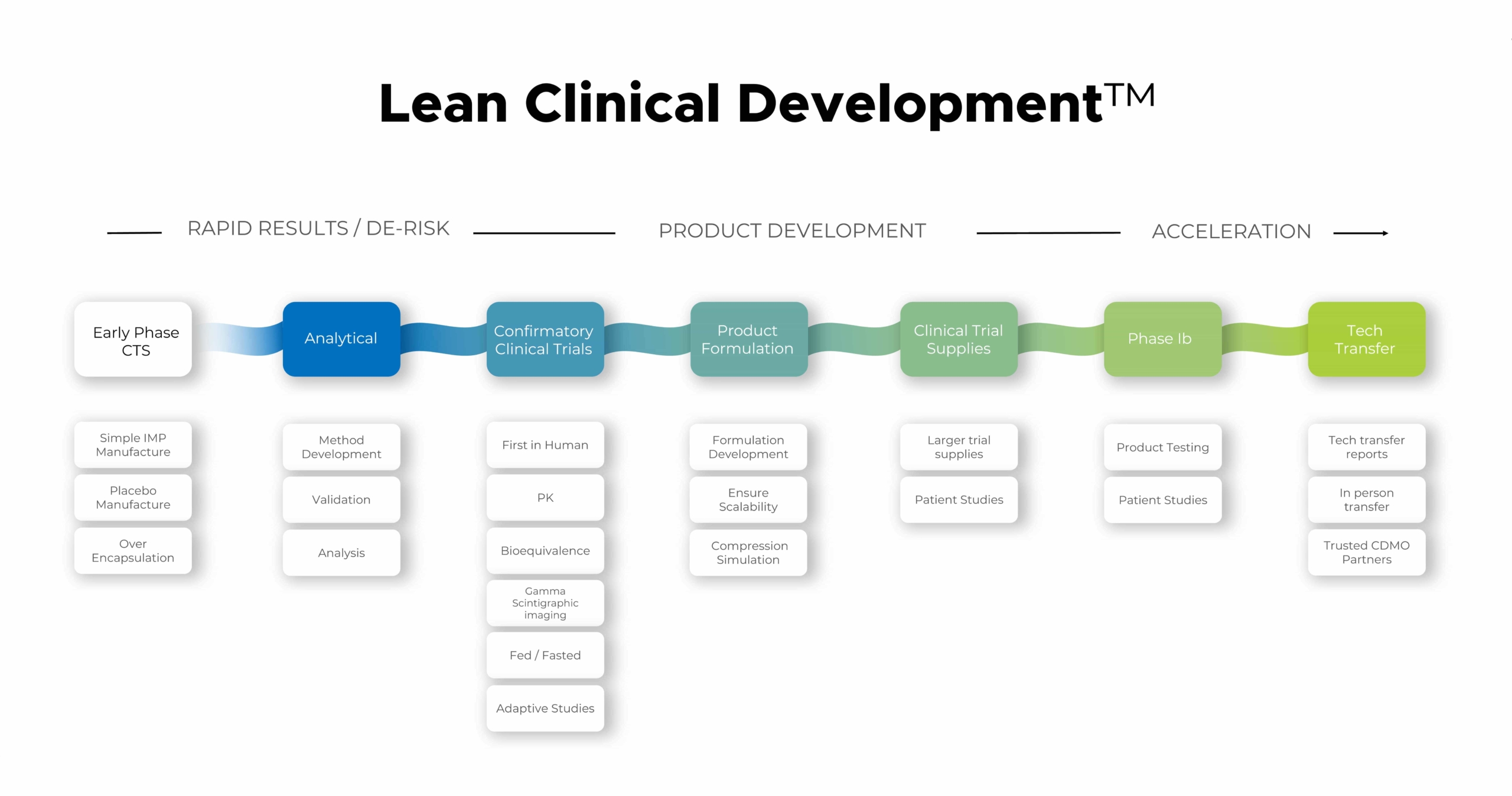
BDD are proud to introduce Lean Clinical Development™. Our single point solution to get Phase 1 clinical data faster by eliminating unnecessary steps. We have revolutionised the path from molecule to clinical results by combining “just in time” manufacture and clinical agility in our bespoke integrated pharmaceutical facilities.
Lean Clinical Development™ at BDD accelerates your project through early formulation and Phase I trials. This modular service allows you to access each step of the pathway independently or you can work with us for the whole journey to Phase II. Our expertise in complex and controlled release, biopharmaceutics, and gamma scintigraphy ensures we address drug delivery challenges early on. With our MHRA-accredited GMP facilities, we offer ‘just in time’ manufacturing of tablets, capsules, and radiolabelled products, streamlining your path to clinical dosing.
Experienced team
BDD has over 25 years’ success conducting early phase confirmatory clinical trials
Founded and led by Science
BDD was founded in 1999 by scientists and has always held great science at the absolute forefront of everything we do.
Proven reliability and track record
BDD has a superb, proven track record working from formulation through clinic across hundreds of happy clients.
Faster
BDD’s Lean Clinical Development approach considerably reduces traditional development pathways.
Industry leading recruitment
BDD have for decades built up a robust and effective volunteer and patient database enabling us to have an industry leading 80.2% screening-to-enrolment rate. Your study starts on time with BDD.