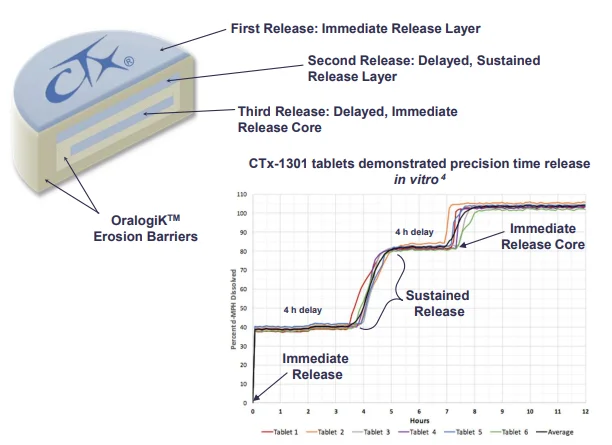
Case Study: Pharmacoscintigraphic and Pharmacokinetic Analysis of CTx-1301, a Novel Tri-modal Oral Formulation for Release of Dexmethylphenidate in Healthy Adults
Abstract
Background: CTx-1301 is a dexmethylphenidate (d-MPH) tri-modal tablet formulation comprised of an immediate release layer, a delayed extended-release layer, and a final immediate-release core designed to provide a fast onset, and ultimately therapeutically active levels with d-MPH lasting 14-16 h. Finally, designing a preparation with a controlled descent of d-MPH was envisioned to minimize the rebound effect and maintain favorable tolerability. The primary focus of the presentation is to describe how the third delayed core performed in terms of where it is delivered in the gut, and how successfully the delivery mechanism achieved the controlled descent.
Methods: A randomized, three-arm, open-label crossover study was performed in 15 healthy volunteers (mean age = 25 years; weight = 77 kg) to establish the PK profile of a novel d-MPH modified-release tablet (CTx-1301) using pharmacoscintigraphic methods. Each volunteer underwent three treatment arms receiving a bi-phasic extended-release d-MPH 10 mg (Focalin® XR) (Treatment A), and CTx-1301 12.5 mg tablets (Treatment B contained a radiolabeled second d-MPH layer, and Treatment C contained a radiolabeled third d-MPH layer). Serial blood samples were taken over a 24-h period after dosing and plasma concentrations of d-MPH were analyzed for all three groups to determine relevant pharmacokinetic parameters including initial and subsequent Cmax and Tmax, as well as AUC(0-8h), AUC(8-24h), and AUC(0-inf) Scintigraphic methods were used to visualize timing and location of release of radiolabeled d-MPH layers from CTx-1301 tablets.
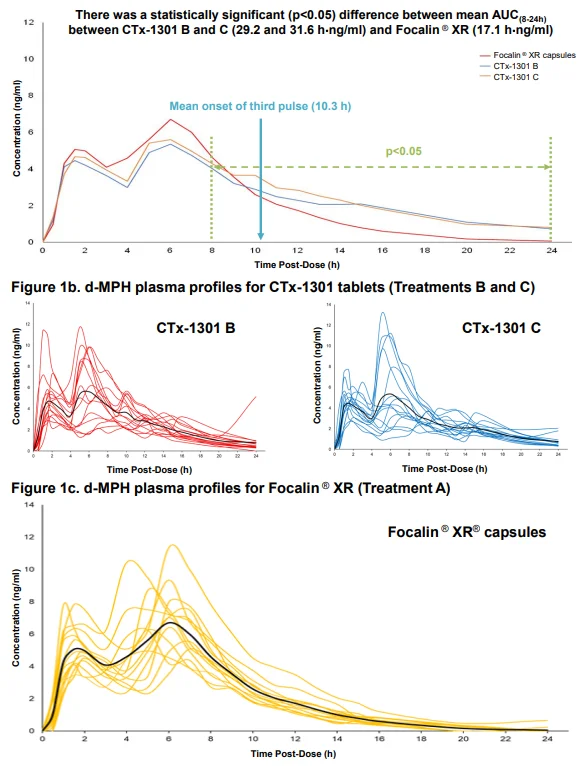
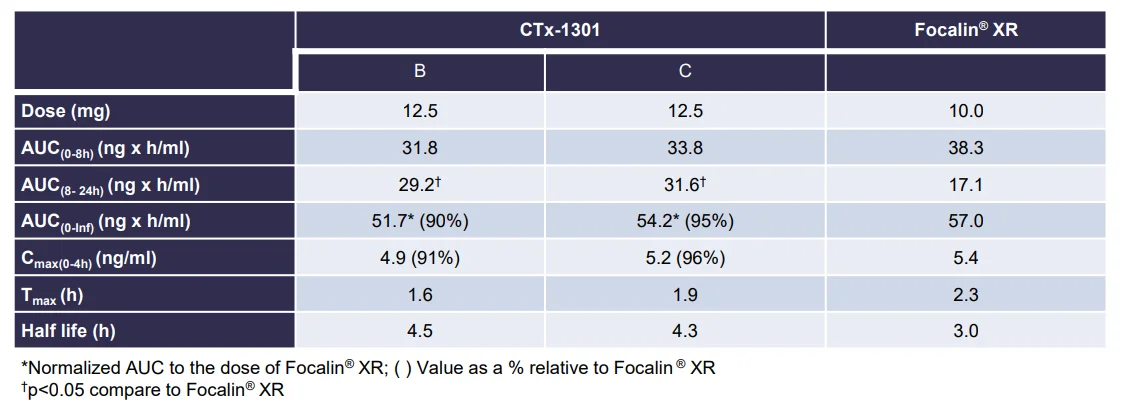
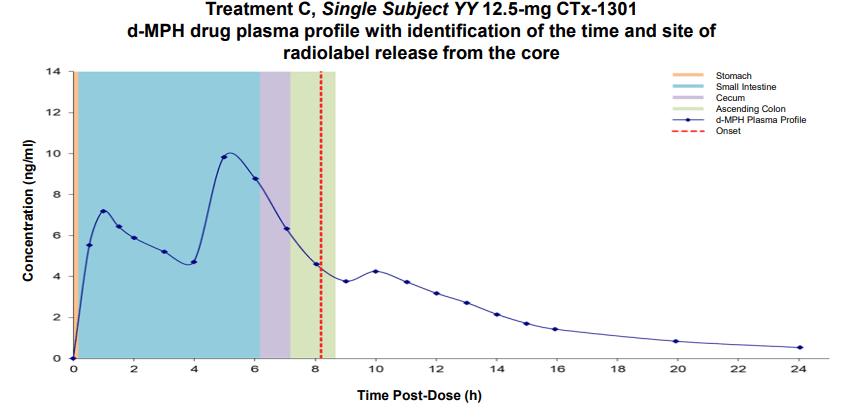
Results: The two CTx-1301 treatment arms demonstrated initial Tmax (0-4h) of 1.6 h and 1.9 h versus 2.3 h for Focalin ® XR and initial Cmax (0-4h) (4.9 and 5.3 vs 5.9 ng.ml1). The terminal half-life was extended by more than an hour in the CTx-1301 treatments (4.5 and 4.3 h) versus Focalin® XR (3.0 h). Scintigraphic measurements determined mean time of release of the CTx-1301 second layer to be 4.7 h, and the
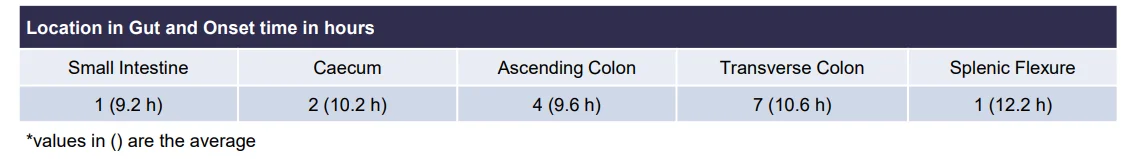
third layer (core) to be 10.3 h There was a statistically significant (p<0.005) difference between mean AUC(8-24h) between CTx-1301 B and C (29.2 & 31.6 h⋅ng/ml) and Focalin XR® (17.1 h⋅ng/ml). Onset of release of the second CTx-1301 layer was visualized in the small intestine (N=8), caecum (N=3), and ascending colon (N=4), while onset of release of the third layer was visualized in the small intestine (N=1), caecum (N=2), ascending colon (N=4), transverse colon (N=7), and splenic flexure (N=1). No serious adverse events were reported following any treatment.
Conclusion: Mean initial Cmax and Tmax values were comparable between the three treatment arms. Second and third delayed-release layers of CTx-1301 were delivered as designed, maintaining blood levels of d-MPH longer than Focalin ® XR resulting in a slower descent of d-MPH. Future investigations will include classroom studies to link the pharmacokinetics and clinical efficacy of CTx-1301, including the rebound effect.