CONCLUSIONS
Polymer encapsulation of indium-111 chloride enabled successful radiolabeling of a large, swelling gastric retentive formulation for gamma scintigraphy evaluation in vitro, preclinically and clinically. The PEIC radiolabel overcame the challenges of premature radiotracer loss in acidic gastric conditions, which compromised assessment with conventional labeling techniques. Enhanced radiolabel retention was achieved by adsorbing indium-111 chloride onto activated charcoal particles and then coating with cellulose acetate polymer, followed by milling to form microparticles that were incorporated into the GRF matrix. This method can potentially improve assessment of gastric retention for other novel oral dosage forms using gamma scintigraphy.
REFERENCES
1. E. A. Klausner, E. Lavy, M. Friedman, and A. Hoffman.
Expandable gastroretentive dosage forms. J. Control. Release
90:143Y162 (2003).
2. J. A. Fix, R. Cargill, and K. Engle. Controlled gastric emptying.
III. Gastric residence time of a nondisintegrating geometric
shape in human volunteers. Pharm. Res. 10(7):1087Y1089 (1993).
3. E. A. Klausner, E. Lavy, M. Barta, E. Cserepes, M. Friedman,
and A. Hoffman. Novel gastroretentive dosage forms: evaluation of gastroretentivity and its effect on levodopa absorption in
humans. Pharm. Res. 20(9):1466Y1473 (2003).
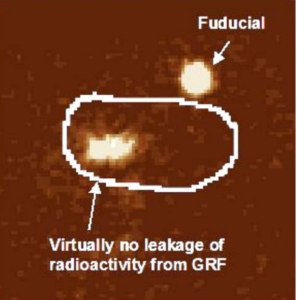
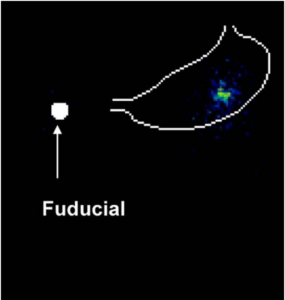
Fig. 8. PEIC radiolabeled GRF in the stomach of healthy human
volunteer, 18 h post dose. Virtually no leakage of the radiolabel was
observed. The stomach outline is based on imaging a co-dosed
99mTechnetium labeled egg.
A Novel Method to Radiolabel GRFs for Gamma Scintigraphy Assessment 703
4. F. Kedzierewicz, P. Thouvenot, J. Lemut, A. Etienne, M.
Hoffman, and P. Maincent. Evaluation of peroral silicone
dosage forms in humans by gamma-scintigraphy. J. Control.
Release 58:195Y205 (1999).
5. W. Webb. Management of foreign bodies of the upper gastrointestinal tract: update. Gastrointest. Endosc. 41:39 (1995).
6. H. Koch. Operative endoscopy. Gastrointest. Endosc. 24(2):65
(1977).
7. H. Kato, M. Nakamura, E. Orito, R. Ueda, and M. Mizokami.
The first report of successful nasogastric coca-cola lavage
treatment for bitter persimmon phytobezoars in Japan. Am. J.
Gastroenterol. 98(7):1662Y1663 (2003).
8. A. Steingoetter, D. Weishaupt, P. Kunz, K. Mader, H.
Lengsfeld, M. Thurnshirn, P. Boesiger, M. Fried, and W.
Schwizer. Magnetic resonance imaging for the in-vivo evaluation of gastric-retentive tablets. Pharm. Res. 20(12):2001Y2007
(2003).
9. Given Imaging (http://www.givenimaging.com)
10. W. Weitschies, R.-S. Wedemeyer, O. Kosch, K. Fach, S. Nagel,
E. So¨ derlind, L. Trahms, B. Abrahamsson, and H. Mo¨ nnikes.
Impact of the intragastric location of extended release tablets on
food interactions. J. Control. Release 108:375Y385 (2005).
11. P. Goethals, A. Volkaert, B. Van Vlem, and R. Vanholder.
Critical evaluation of the chemical standardization procedure for
measuring gastric emptying of solids. J. Label. Compd. Radiopharm. 45:1091Y1096 (2002).
12. R. J. Kowalsky, and S. W. Falen. Radiopharmaceuticals in
Nuclear Pharmacy and Nuclear Medicine, 2nd ed. American
Pharmacists Association, Washington, DC, 2004.
13. J. W. Ayres. Expandable gastric retention device. US Patent
Application #20040219186-A1, Nov. 4, 2004.
14. J. D. Gardner, A. A. Ciociola, and M. Robinson. Measurement
of meal-stimulated gastric acid secretion by in-vivo gastric
autotitration. J. Appl. Physiol. 92:427Y434 (2002).
15. M. P. Williams, J. Sercombe, M. I. Hamilton, and R. E. Pounder.
A placebo-controlled trial to assess the effects of 8 days of
dosing with rabeprazole versus omeprazole on 24-h intragastric
acidity and plasma gastrin concentrations in young healthy male
subjects. Aliment. Pharmacol. Ther. 12:1079Y1089 (1998).
16. V. Pai, M. Srinivasarao, and S. A. Khan. Evolution of
microstructure and rheology in mixed polysaccharide systems.
Macromolecules 35:1699Y1707 (2002).
17. S. J. J. Debon, and R. F. Tester. In vitro binding of calcium, iron
and zinc by non-starch polysaccharides. Food Chem.
73(4):401Y410 (2001).
18. M. D. Burke, J. O. Park, M. Srinivasaro, and S. A. Khan.
Diffusion of macromolecules in polymer solutions and gels: a
laser scanning confocal microscopy study. Macromolecules
33(20):7500Y7507 (2000).
19. A. Martin. Physical Pharmacy, 4th ed. Lea and Febiger, Philadelphia, 1993.
20. A. Keshavarzian, W. E. Barnes, K. Bruninga, B. Nemchausky,
H. Mermall, and D. Bushnell. Delayed colonic transit in spinal
cord-injured patients measured by indium-111 Amberlite scintigraphy. Am. J. Gastroenterol. 90:1295Y1300 (1995).
21. R. Cargill, L. J. Caldwell, K. Engle, J. A. Fix, P. A. Porter, and C.
R. Gardner. Controlled gastric emptying. I. Effects of physical
properties on gastric residence times of nondisintegrating geometric shapes in beagle dogs. Pharm. Res. 5:533Y536 (1988).
22. R. Cargill, K. Engle, C. R. Gardner, and J. A. Fix. Controlled
gastric emptying. II. In vitro erosion and gastric residence times of
an erodible device in beagle dogs. Pharm. Res. 6(6):506Y509 (1989).
Acknowledgements


CONTACT INFORMATION:
980 Great West Road, Brentford, Middlesex, TW8 9GS, United Kingdom, Tel: +44 20 8047 5000 info@gsk.com
BDD Pharma Ltd, Glasgow Royal Infirmary, 84 Castle St., Glasgow G4 0SF, UK: +44 (0)141 552 8791; enquiries@bddpharma.com