Revolutionising Respiratory Formulation and Device Development: The Impact and Future of Gamma Scintigraphy in Pulmonary Drug Delivery
Introduction:
In the development of respiratory and pulmonary medicines, the efficiency of drug delivery systems is of paramount importance. Precise and targeted delivery of medication to the lungs is a complex yet crucial aspect of treating respiratory conditions. Gamma scintigraphy is a well established imaging technique, offering unparalleled insights into the performance of drug formulations. This article explores the profound impact of gamma scintigraphy in revolutionizing respiratory and pulmonary drug development.
What is Gamma Scintigraphy?
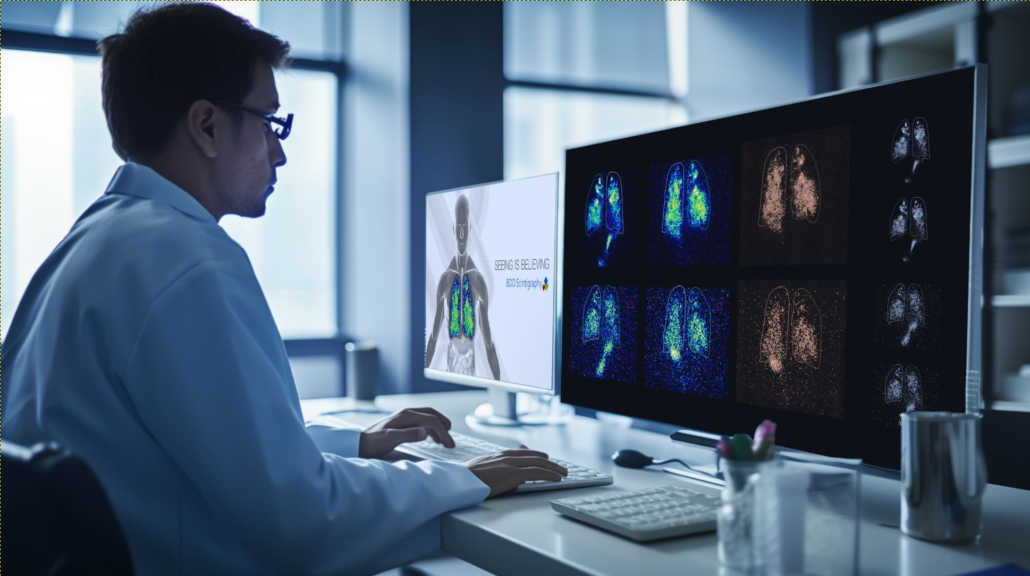
Gamma scintigraphy is a sophisticated diagnostic imaging technique that utilises gamma rays to capture dynamic images of the performance of a drug formulation within the body. It was initially developed as a tool in nuclear medicine but has since become a powerful asset in the study of respiratory drug delivery. The process involves administering a radiolabelled drug formulation, and then tracking the gamma radiation emitted as the labelled formulation is administered to the respiratory system. This technique allows for non-invasive, real-time visualisation of the formulations deposition and dispersion within the lungs.
Advantages and uses of Gamma Scintigraphy for Respiratory and Pulmonary Formulations:
- Non-Invasive Visualisation: Gamma scintigraphy enables the determination and quantification of drug distribution patterns within the lungs without any surgical intervention, making it an ideal exploratory development tool.
- Real-Time Monitoring: It offers the unique ability to monitor the drug delivery process in real time, providing immediate feedback on the performance of drug formulations.
- Precision and Accuracy: The technique is highly accurate, allowing for detailed analysis of drug deposition and distribution, crucial for optimising and proving formulation or device performance. Scintigraphy is invaluable in understanding the percentage dose delivered to the whole lung, central lung or peripheral lung and calculating the C/P index.
- Comparative Performance: This technique allows you to understand the comparative performance of both devices and formulations in man to help determine your lead formulation or device to proceed with in further, more extensive clinical trials.
Using Gamma Scintigraphy to Assess Mucociliary Clearance:
Mucociliary clearance is a natural lung function which forms part of the innate immune response, protecting the lungs from airborne pathogens. The importance of this function is underlined by severely debilitating diseases such as cystic fibrosis or bronchiectasis in which clearance is impaired. Mucociliary clearance studies involve administering inhaled radiolabel to healthy volunteers / patients, and then taking a series of images with the gamma camera over different time points to quantify the rate at which the radiolabel is cleared, providing a measurement of the mucociliary clearance rate.
These studies can be used to either:
- Confirm that there is no change in mucociliary clearance following treatment . With recent regulatory guidance and the current industry shift towards more eco-friendly propellants, a gamma scintigraphy study can prove that the introduction of a new propellant to your pMDI has no negative impact on mucociliary clearance.
- For treatment efficacy. A gamma scintigraphy study can be used to demonstrate an improvement in mucociliary clearance for mucous obstructive disease therapies, such as Cystic Fibrosis, chronic obstructive pulmonary disease (COPD), primary ciliary dyskinesia (PCD) and Non-Cystic Fibrosis Bronchiectasis (NCFB).
Case Study:

Comparative Analysis With Other Imaging Techniques:
While techniques like MRI and CT scans offer detailed anatomical images, gamma scintigraphy stands out for its ability to specifically track the movement and distribution of drug formulations within the respiratory system. Unlike X-rays, which provide static images, gamma scintigraphy offers dynamic, real-time video visualisation, making it more suitable for studying the behaviour of inhaled medications.
Future Trends and AI Innovations:
The future of gamma scintigraphy looks promising with the integration of AI, enhancing image resolution, reducing radiation exposure and improving diagnostics. Innovations in radiolabelling and digital imaging technologies are expected to elevate its capabilities. AI-driven image analysis can identify trends and anomalies in images, leading to more accurate interpretations and early detection of diseases like cancer and pulmonary disorders. It enables quantitative analysis and personalized treatment planning by automating radiotracer uptake quantification and analysing patient-specific data.
AI can also play a crucial role in reducing radiation doses, optimising workflow, and integrating gamma scintigraphy data with other modalities for comprehensive evaluation. It’s useful in training healthcare professionals and in pharmaceutical research for assessing drug efficacy, particularly in targeted delivery systems. This integration signifies a leap in medical imaging, offering more precise, efficient, and tailored solutions. As AI evolves, its application in gamma scintigraphy is expected to reveal new diagnostic and therapeutic avenues, with BDD remaining at the forefront of these developments.
Conclusion:
Gamma scintigraphy has established itself as an invaluable tool in the development and optimisation of respiratory and pulmonary drug formulations. Its ability to provide detailed, real-time insights into drug distribution within the lungs is unrivalled, offering critical data that can significantly enhance the efficacy of respiratory therapies. As pharmaceutical technology continues to evolve, the role of gamma scintigraphy is set to become even more pivotal in the quest for effective and efficient respiratory treatment options.
Join BDD at DDL – BDD’s Calum Stevens and Darren Quinn will be at DDL in Edinburgh answering questions about how gamma scintigraphy can help you to accelerate development of your respiratory products and advance your product to market.